Pulmonary Embolism Trajectory Prediction Using Machine Learning
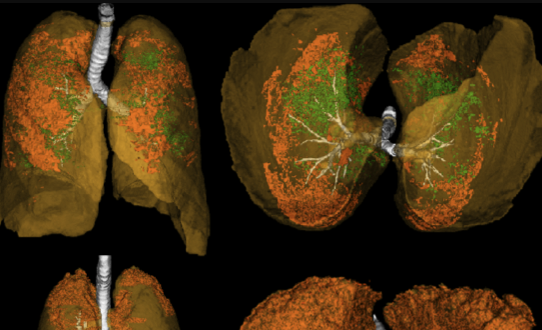
Ongoing Master’s thesis research at Brigham and Women’s Hospital Applied Chest Imaging Laboratory under Dr. Syed Moin Hassan.
Pulmonary embolism (PE) remains a leading cause of cardiovascular mortality, affecting hundreds of thousands of patients annually in the United States. While clinical guidelines provide standardized approaches to acute PE management, patient trajectories following diagnosis vary considerably—from complete recovery to recurrent events, chronic thromboembolic disease, or mortality. Understanding which patients face elevated long-term risks, and why, represents a critical gap in current PE care.
As a Master’s student at Brigham and Women’s Hospital in the Applied Chest Imaging Laboratory, I’m investigating pulmonary embolism trajectories using machine learning approaches under the mentorship of Dr. Syed Moin Hassan, MD.
Research Objectives
This ongoing thesis work aims to leverage computational methods to identify patterns in PE patient outcomes that may not be apparent through traditional clinical risk stratification. By integrating imaging data, clinical variables, and longitudinal patient records, the goal is to develop models that can better characterize post-PE trajectories and potentially identify patients who may benefit from intensified monitoring or modified treatment strategies.
Key Research Questions
- Can machine learning models identify distinct trajectory patterns among PE patients beyond current risk stratification schemes?
- Which clinical and imaging features most strongly predict long-term outcomes following acute pulmonary embolism?
- How do patient characteristics at presentation relate to recovery trajectories, recurrence risk, and other long-term complications?
Methodological Framework
The research involves several key components:
Data Integration: Combining chest imaging findings, electronic health record data, and clinical outcomes to create comprehensive patient profiles that capture both the acute presentation and longitudinal course.
Feature Engineering: Identifying and extracting clinically relevant variables that may contribute to trajectory prediction, including imaging-based measurements, laboratory values, comorbidities, and treatment patterns.
Model Development: Applying supervised and unsupervised machine learning techniques to classify trajectory patterns and assess predictive performance against established clinical risk scores.
Clinical Validation: Ensuring that computational findings align with clinical knowledge and evaluating whether model predictions offer actionable insights for patient management.
Clinical Context & Significance
Current PE risk stratification focuses primarily on short-term mortality and hemodynamic stability during the acute phase. However, patients who survive the initial event face variable long-term outcomes including:
- Recurrent venous thromboembolism
- Chronic thromboembolic pulmonary hypertension (CTEPH)
- Post-PE syndrome with persistent dyspnea and functional limitation
- Bleeding complications from anticoagulation therapy
Better understanding of these trajectories could inform decisions about anticoagulation duration, surveillance imaging strategies, and early intervention for patients at risk of chronic complications.
Applied Chest Imaging Laboratory
The Applied Chest Imaging Laboratory focuses on translating advanced imaging analytics into clinical applications for thoracic diseases. The lab’s interdisciplinary approach combines radiological expertise with computational methods to address questions in pulmonary vascular disease, interstitial lung disease, and other chest pathologies.
Current Status
This work is in active development as part of my Master’s thesis. Initial phases involve data curation, establishing analytical pipelines, and preliminary model development. As the research progresses, findings will be refined through iterative validation and clinical input.
The complexity of PE trajectories—influenced by individual patient factors, treatment variations, and unmeasured confounders—necessitates careful methodological consideration. This work aims to contribute incremental progress toward more personalized approaches to PE management, recognizing that machine learning offers tools for pattern recognition but requires rigorous clinical context to generate meaningful insights.
Acknowledgments
I am grateful to Dr. Syed Moin Hassan for his mentorship and guidance on this project. His expertise in thoracic imaging and clinical pulmonary vascular disease provides essential perspective for ensuring this computational work remains grounded in patient care realities.
Thank you to the Applied Chest Imaging Laboratory at Brigham and Women’s Hospital for providing the infrastructure and collaborative environment that makes this research possible.
This research represents ongoing work toward a Master’s thesis. Updates on findings and methodological developments will be shared as the project progresses. The ultimate goal is to contribute evidence that may inform more nuanced approaches to pulmonary embolism care and improve long-term outcomes for this patient population.
